Abstract
Mutations in DNA damage regulators and epigenetic modifier genes including those in DNA methyltransferase 3A (DNMT3A) are common in pre-malignant clonal hematopoiesis (CH) and are recurrent in myeloid malignancies such as myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). While loss-of-function (LOF) mutations in DNMT3A are predominant in CH, single amino-acid substitutions at residue 882, usually from an arginine to a histidine or cysteine (DNMT3A R882H / DNMT3A R882C), are strongly enriched in AML, suggesting a role in promoting pre-malignant clonal evolution. In this study, we compared and contrasted the effects of DNMT3A R882 and LOF on hematopoietic stem cell (HSC) function by investigating self-renewal capacity in vitro and bone marrow repopulation potential in vivo in a genetic mouse model.
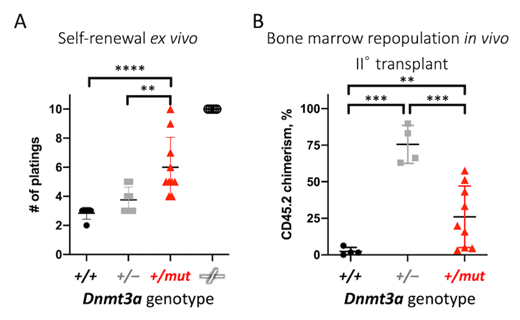
Bone marrow (BM) from mice conditionally expressing Dnmt3a R878H (equivalent to human DNMT3A R882H) from the endogenous locus, denoted as +/mut, was subjected to colony forming unit (CFU) assays in semisolid media (MethoCult GF M3434, Stem Cell Technologies) to detect hematopoietic progenitors with serial replating every 14 days as a surrogate measure of stem cell self-renewal. Dnmt3a +/mut cells were compared to bone marrow derived from mice with heterozygous or homozygous Dnmt3a knockout (+/- and -/-, respectively), or wild-type control (+/+). Dnmt3a +/- BM cells showed a trend towards a slight increase in the number of replatings compared to wild-type controls (2.83±0.41 in +/+ vs 3.75±0.89 in +/-, n=6,8, Mann-Whitney test p=0.053). In contrast, Dnmt3a +/mut BM occasionally resulted in a significant gain of self-renewal with replating for 6+ passages observed in 4/10 independent trials (6.00±2.06 in +/mut, n=10, p=0.001 vs +/+), yet failed to reach continuous replating of complete Dnmt3a loss that served as a positive control (terminated at passage 10, n=5). Substantial heterogeneity in the Dnmt3a +/mut self-renewal capacity ex vivo was further reflected by increased statistical variance (Welch's F-test p=0.037 compared to +/-).
To extend these studies to the in vivo setting, we performed gold-standard serial competitive bone marrow repopulation assays. Here, 250 FACS-sorted Lineage -Sca1 +cKit +CD48 -CD150 + (LSK-SLAM) test cells marked with CD45.2 were mixed with 0.25x10 6 unfractionated wild-type competitor bone marrow cells marked with CD45.1 and engrafted into pre-conditioned congenic recipients (CD45.1). Peripheral blood CD45.2/CD45.1 chimerism is used as a readout for the ability of test cells to reconstitute hematopoiesis; self-renewal potential is assessed via serial retransplantation. In this setting, Dnmt3a +/+ cells showed a decline in their ability to reconstitute hematopoiesis in secondary transplant recipients, while Dnmt3a haploinsufficiency resulted in a competitive advantage over wild-type (CD45.2 chimerism 2.4±2.7% in +/+ vs 75.6±13.0% in +/-, n=4, Welch's t-test p=0.001). In contrast, Dnmt3a +/mut cells displayed a high degree of variability in their long-term stem cell function in some cases yielding high levels of chimerism (CD45.2 >40% in 3/9 recipients) and in other cases not (26.0±21.0%, n=9, p=0.01 vs +/+), in agreement with CFU assays.
Collectively, these data suggest that the DNMT3A R882 mutations endow normal HSCs with phenotypic heterogeneity yielding a population of cells with variable stem cell function, some of which may have higher fitness thus accelerating clonal evolution towards malignancy. These findings will be built upon in future experiments aiming to identify underlying molecular mechanisms and to improve our understanding of the pathophysiology and prognostication of clonal hematopoiesis.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal